Overview
|
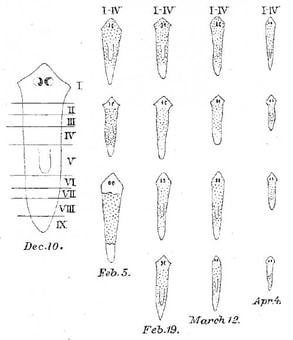
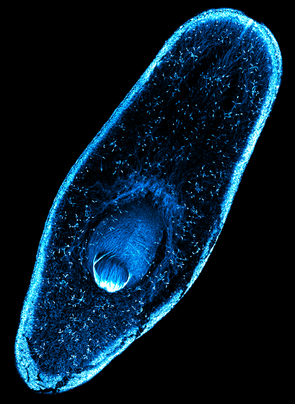
Planarians are flatworms with an astounding regenerative capacity. As described by Nobel Prize winner and University of Kentucky alumnus T.H. Morgan, even a small fragment of an adult worm can regenerate into a new, fully-formed animal! Planarian regeneration relies on an abundant population of adult stem cells, making them an excellent model for studying both in vivo differentiation and animal regeneration. Planarian stem cells are both highly similar to other types of stem cells and yet also unique. Much like pluripotent stem cells in early mammalian embryos, they both self-renew their own population AND differentiate into all the cell lineages that comprise a mature worm. Yet unlike mammalian stem cells, which are only pluripotent very briefly during early embryogenesis, planarian stem cells maintain their "stemness" indefinitely. In the Duncan lab, we are particularly interested in how regulation of the planarian genome and chromatin state contribute to these powerful biological features. |
Projects
mll1/2 function in regenerationMLL1 and MLL2 are chromatin modifying enzymes best known for regulating the expression of Hox genes, transcription factors that specify the animal body plan during development. Interestingly, RNAi of mll1/2 in the planarian species Schmidtea mediterranea (S.med) does not prevent, or grossly distort, regeneration after head or tail amputations. We hypothesized that the mll1/2(RNAi) phenotype, or lack of phenotype, depends on the type of injury used to induce regeneration. Specifically, we hypothesize that loss of mll1/2 will impact regeneration more significantly when the injured tissue must reestablish a new body plan i.e., in lateral tissue fragments. We further aim to determine if the reestablishment of the planarian body plan in lateral tissue fragments requires activation of hox genes or other gene networks. |
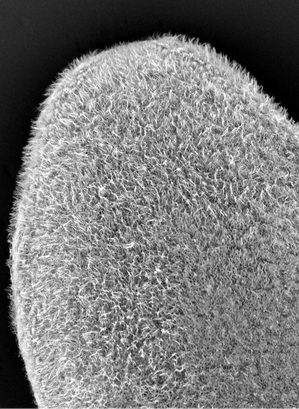
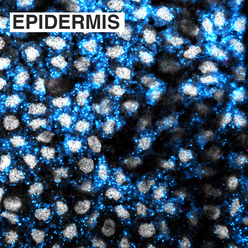
cilia, signaling, and Stem cellsThe most obvious phenotype upon RNAi knockdown of mll1/2 in planarians is abnormal motility, or "inch-worming". mll1/2(RNAi) worms progressively lose their epidermal cilia and analysis of their chromatin state in revealed that MLL1/2 indeed targets cilia gene loci, marking them with histone H3 lysine 4 trimethylation (H3K4me3), a modification known to correlate with gene activation. Unexpectedly, SmedMLL1/2 targets cilia gene loci in planarian stem cells as well as in differentiated cells, even though planarian stem cells do not express cilia genes or bear ciliated structures. We hypothesize that MLL1/2 targeting serves to "prime" these loci for later expression i.e., upon differentiation into ciliated cell types. We are interested in understanding the mechanism(s) of this "priming". Is it an autonomous or non-autonomous (e.g., signal-dependent) mechanism? How does SmedMLL1/2 target cilia genes (and other loci) so specifically, particularly when these loci are not being actively transcribed? |
silencing of mll1/2 targets in Stem cellsAnother aspect of SmedMLL1/2 priming we want to understand is how the expression of these target genes is regulated. Given that MLL1/2 catalyzes a post-translational modification normally found on actively transcribing genes (histone H3 lysine 4 trimethylation), it's interesting that SmedMLL1/2 targets specific non-expressed and/or lowly-expressed genes in planarian stem cells. Moreover, the genes targeted by SmedMLL1/2 in stem cells are highly expressed in differentiated cells. We are interested in the mechanisms controlling these divergent states. How are SmedMLL1/2 genes kept inactive in planarian stem cells? Is this silencing required for proper differentiation into specific lineages? Chromatin "bivalency", a state in which a genome locus is marked by both an activating and repressive modification, is one possible mechanism. However, although bivalency is highly-studied in cultured mammalian stem cells, its status and functional relevance are not well established in organismal contexts. In this project, we aim to address whether chromatin state — bivalency or others — plays an important role in silencing SmedMLL1/2 target genes and their subsequent activation during planarian stem cell differentiation. |